An historical memoir in honour of Maurice Wilkins
1916-2004

|
Maurice Hugh Frederick Wilkins was born in Wellington, New Zealand on
December 15, 1916. His father had moved there from Dublin in 1913 to
practice medicine and the family did not return until 1923 thereby missing
the horrors of World War I and the coincidental troubles in Ireland during
and after the war.
|
Maurice died on October 5, 2004 at Blackheath, London
where he had resided for the last half of his long life. In between there
was a good education at King Edward's School in Birmingham and at St John's
College, Cambridge where he did not get a good enough degree to be invited
to stay on and do research in Physics as he might have wished.
The personal and professional consequences were profound. Exploiting his St
John's network he got a place at Birmingham where his old tutor, Mark
Oliphant, had recently (1937) become Professor and J. T. Randall, newly
arrived with his Warren Research Fellowship of the Royal Society, was
looking for recruits to do research on the luminescence of solids. The
Oliphant connection led to Maurice's wartime participation in the Manhattan
Project (1944-5) and his brief first marriage. The Randall connection
provided lifelong scientific patronage on a munificent scale as Sir John
moved on from his co-invention at Birmingham of the radar-stabilising cavity
magnetron to the Chair of Natural Philosophy at St Andrews, then the
Wheatstone Chair of Physics at King's College London and the simultaneous
Directorship of the MRC Biophysics Unit there. Randall, the abrasive
impresario, had to build and develop two new departments (Biophysics as well
as Physics) during his time at King's and throughout used Maurice as an
emollient deputy, a congenial and important role that he resented only
occasionally as he progressed from assistant to deputy director of the MRC
Unit, the Chair of Molecular Biology, and eventually succession to the
directorship on Randall's retirement (1970).
Along the way something far more exciting happened: Maurice encountered
DNA, played a key role in unveiling and establishing its double helical
structures and the related ones of some RNAs. For these achievements he was
elected to the Royal Society (1959), received the 1960 Albert Lasker award
(made to Wilkins, Crick and Watson in that order), and finally in 1962
shared (also with Watson and Crick) the Nobel Prize for Physiology and
Medicine. By this time Maurice had re-married and with his new and growing
family might have lived happily ever after had not Jim Watson published a
provocative, best-seller about the provenance of the DNA double helix. This
spawned other hopeful literary monsters in which Maurice, the unassertive
third man of the double helix, became a convenient vaudeville villain for
those seeking posthumous recognition of another King's physical scientist,
R.E.Franklin, who also had contributed to X-ray diffraction studies of DNA.
It has to be understood that the MRC Biophysics Unit at King's was not
intended to study macromolecular structures. Its chosen tools would be
physical (optical and electron microscopy and spectroscopy), but the targets
of its investigations would be supra-molecular (chromosomes, cells and
tissues, and motile elements like cilia and flagella). Consequently there
was no early investment in X-ray diffraction equipment or personnel. The
Wheatstone Laboratory's diffraction expert, A.R. Stokes, was very much a
physicist and not a chemical crystallographer. In fact it is not unfair to
say that there was a pervasive suspicion of crystals. These were tombs for
dead molecules but physicists who had become biophysicists preferred to be
seen to be studying more vital systems. It says a great deal for Maurice
Wilkins' insight that he was not only one of the first to accept that DNA
was indeed the genetic material but on discovering that its gels could be
ordered at the molecular level he at once decided to abandon his optical
microscopes for the higher resolution probe of X-ray diffraction.

|
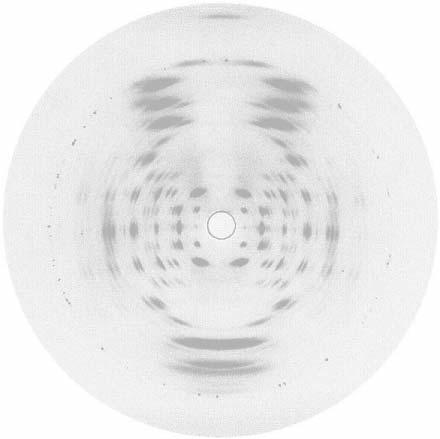
Fig.1. A-DNA diffraction with the fiber tipped into the X-ray beam to record
the 0,0,11 reflexion dignostic of the 11-fold screw symmetry of the
molecules.
|
Despite the local practical difficulties he and R. G. Gosling were able to
produce by the summer of 1950 a well-oriented and polycrystalline specimen
of what we now call A-DNA. It was an early version of its diffraction
pattern (Fig.1) shown by Maurice at a meeting in Naples in the Spring of
1951 that so excited J. D. Watson with the prospect that gene structures
might be simple and crystallisable.
Stokes and Gosling determined the unit
cell dimensions of A-DNA (a=22A, b=40A, c=28A, ?=970) and
accurately assigned the monoclinic space group C2. These dimensions imply
that in projection down the fibre axis the polymer molecules are packed on
an approximately hexagonal net of spacing ~22A and the space group symmetry
implies that the evenly spaced molecules would have to consist of pairs of
chains related by diad axes in the plane of the net.
In retrospect it is difficult to imagine a committed and well-trained
crystallographer looking at space group no.5 in International Tables and not
concluding that the A-DNA unit cell would contain 4 quasi-identical
polynucleotide chains, diadically paired and packed like a bundle of
cylinders of 22A diameter. Of course the bundled chains could not be
cylinders exactly but spirals with 11-fold screw symmetry as indicated by
the absence of meridional X-ray reflexions until the appearance of the
diagnostic 0,0,11 reflexion that is so prominent at the top of Fig.1. As
every crystallographer would know: an 11-fold screw axis could not be a
crystal symmetry and therefore it would have to be a molecular
property !
If DNA were indeed the genetic material then the information it contained
would have to be complex at some level of resolution but here again
classical crystallographers should not have been dismayed by the apparent
simplicity of the A-DNA crystal structure. Crystalline minerals excited much
attention both before and after the discovery of X-ray diffraction . The
bewildering complexity of their chemical compositions was a challenge until
it could be shown by X-ray crystallography that a relatively few
three-dimensional structural motifs of alumina and silica could accommodate
a wide variety of chemical variation. DNA presented an analogous challenge:
how might the constituents of chemically diverse polynucleotides form
isomorphous components that might vicariously replace one another in a
simple regular structure like a helix. This was the problem addressed
directly by the biologist J.D.Watson and solved by his discovery of
base-pairing after some crucial advice about tautomerism from the chemical
crystallographer Jerry Donahue. Of course a demonstration model had to be
built to show that Watson's base-pairs could be accommodated in a double
helical cage with the correct overall dimensions but it is fair to say that
such niceties would be of little interest to molecular biologists for whom
the duplex nature of DNA and the complementary base pairing would be the key
revelations.
All this happened at Cambridge while the London DNA effort was taken on a
bizarre detour into the desert of crystallographic orthodoxy by recruitment
of R.E. Franklin, a physical chemist with just enough X-ray diffraction
education obtained while studying coal and coke to be full of wise saws and
modern instances concerning X-ray structure determination in general.
Pre-war methods were out. Too often these had used heuristic methods to
produce preliminary models of unit cell contents from which were obtained a
preliminary set of X-ray phases that were slowly improved by a succession of
Fourier syntheses of electron density and sometimes the introduction of yet
more chemical insights. By 1950 X-ray crystallography was on the threshold
of its robotic, triumphalist stage: with better computational methods and
more sophisticated diffraction theorems, number-crunching of the intensities
alone would solve the phase problem and produce structures needing no
further authentication because no chemical prejudices had tainted their
genesis. More experienced experimentalists might prefer to retain a choice
of horses for courses and and give priority to getting the right answer
rather than to the use of currently correct methods. This kind of thinking
was now anathema at King's.

|
Fig.2. B-DNA diffraction indicating 10-fold screw symmetry and an overall
structure very different in detail from that of A-DNA.
|
Another unhelpful contribution involved a second allomorph of DNA, B, which
can also be uniaxially oriented and persuaded to be polycrystalline in
fibers (Fig.2) which have the appropriate combination of hydration and
retained salts. Preliminary experiments by Franklin suggested that A-DNA was
a 'dry' form although later polymer studies and current oligonucleotide
crystal structures show that A-DNA-like structures are just as hydrated as
B-like duplexes.
But at the time the erroneous 1950s conclusion caused A-DNA
with its straightforward crystal symmetry to be relegated to the role of a
laboratory artefact while much energy was diverted to crystallizing B-DNA,
the 'wet' and therefore more 'biological' form.

|
Fig.3. Diffraction from a fiber containing 12-fold RNA helices with
conformations similar to A-DNA.
|
Only when RNA duplexes were
discovered to have A-like conformations (Fig.3) was A-DNA rehabilitated as a
canonical structure.
The Watson and Crick eureka at Cambridge must have disappointed Maurice at
the time but no one who knew him well would have expected him to be other
than pleased with the outcome . He certainly was more committed to getting
the right answer than to following fashionable procedures. It was ironic
therefore that his next role in the DNA saga was the problem of
authenticating the Watson-Crick hypothesis, and doubly ironic that a subtle
property of A-DNA was the ghost in the machine. The stereochemically
reasonable model that Crick and Watson built to reinforce the plausibility
of their conjecture was designed to be a model of B-DNA. Such was their
attention to precise detail that the 5-membered deoxyribose rings in their
model not only had accurate bond lengths and angles but they also were
puckered and not planar as observed in Furberg's pioneering crystal
structure of the nucleoside cytidine at Birkbeck. There are essentially two
ways in which deoxyribose rings can be puckered, C3'-endo and C2'-endo. Both
are observed in polynucleotide duplexes; the former in A-like structures,
the latter in B-like structures. The macroscopic consequences of these local
conformational differences are quite profound. A-type structures have their
base-pairs about 4A nearer the surface of their double helices than B-type
structures and therefore have a deep groove and a shallow groove in contrast
to B-DNA's more similar grooves. None of this was fully and explicitly
understood until many years later so it was especially unfortunate that
Furberg's cytidine had the C3'-endo-puckered rings appropriate for A-DNA but
not for B!
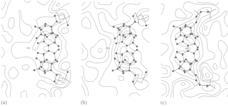
Fig.4.(a) The electron density distribution in the plane of an (average)
Watson-Crick base-pair obtained with diffraction amplitudes for B-DNA and
phase angles calculated from the original Crick-Watson demonstration model.
The image shows not only the (expected) low resolution but also a poor fit
with the model.
(b) The corresponding difference map (with positive density in blue and
negative density in red) reveals the major geometrical flaw in the model is
the position of the base-pairs relative to the helix axis.
(c) A model with the correct deoxyribose conformations and other refinements
shows a better fit with the new electron density map.
|
Thus in 1953, Franklin having left King's for Birkbeck, Maurice Wilkins was
once again in sole possession of the DNA diffraction problem but with a new
and agonizing twist. There now existed a stereochemically entirely plausible
structure for B-DNA that rationalized a great many biochemical observations
and clearly suggested how nucleic acids might function biologically, yet
this attractive structure provide X-ray intensities profoundly at odds with
those observed. The R = 90% discrepancy was nearly twice as bad as that
which textbook theory predicted for a completely wrong structure. Such a
discordance was too provocative to be ignored but it was to take nearly a
decade of improvements in computation, in preparing well-oriented and
polycrystalline specimens, in perfecting X-ray cameras for the special needs
of fiber diffraction, and in developing new methods of structure refinement
before the structures of DNA were fully refined and brought into concordance
with all the diffraction data. There was however an additional dividend from
Maurice's investment: there could now be rapid analyses not only of fibrous
DNAs but also of RNAs and many other spiral structures found with peptide
and carbohydrate polymers that did not form single crystals but were of
biological or industrial importance.
Maurice Wilkins' early acceptance of DNA as the genetic material and his
recognition that it had structures that could and should be tackled by
X-ray diffraction analyses, not necessarily under his exclusive control, was
important in ensuring that the essence of DNA's structure was discovered as
early as it was. His success in resolving patiently and effectively all the
technical problems, great and small, that arose unpredictably in the course
of his own work on DNA and RNA was substantial. His pacific acceptance of
the slings and arrows that unjustly assail those involved in momentous
enterprises was typical and showed a life that had a certain style as well
as much substance.
Struther Arnott
Note from the webeditor:
- This article was published on page 24 of 'Crystallography News' no
92 March 2005
- A bibliography
concerned with the discovery of DNA is on this website
- Celebration of 50th
anniversary of the discovery of the structure of DNA





